
It’s Time To Start Living Again
Is ME/CFS or Long COVID robbing you of the life you deserve?
Your life matters. You matter. You deserve powerful solutions, administered with precision, backed by robust scientific evidence and clinical proof.
Oxaloacetate CFS is a medical food clinically proven to lower fatigue in patients with Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.

Tired Of Being Tired?
New research findings are helping CFS patients take their lives back. Will you be next?
Did you know that Oxaloacetate (OAA) levels are significantly lower in ME/CFS patients?
Enter your email below for a free scientific report with actionable data on:
- Low blood serum Oxaloacetate (OAA) levels in CFS patients
- Dietary Oxaloacetate and Medical Foods for CFS
- The latest peer-reviewed clinical trial data on Oxaloacetate for ME/CFS and Long COVID
- Analysis of the latest research by leading CFS doctors and researchers

Groundbreaking Clinical Trial Data
Oxaloacetate CFS is changing the lives of ME/CFS patients in more than 20 countries worldwide.
As more research is completed, Oxaloacetate CFS is getting the attention of ME/CFS patient advocacy groups, doctors and healthcare practitioners, specialty clinics, scientific journals and medical researchers alike.
Dr. Kaufman Presents
The Groundbreaking Research on Oxaloacetate for ME/CFS and Long COVID
David Lyons Kaufman, MD is a world-renowned expert in ME/CFS and fierce patient advocate. He is the founder of the Center for Complex Diseases and a member of Stanford University’s ME/CFS Research Center.
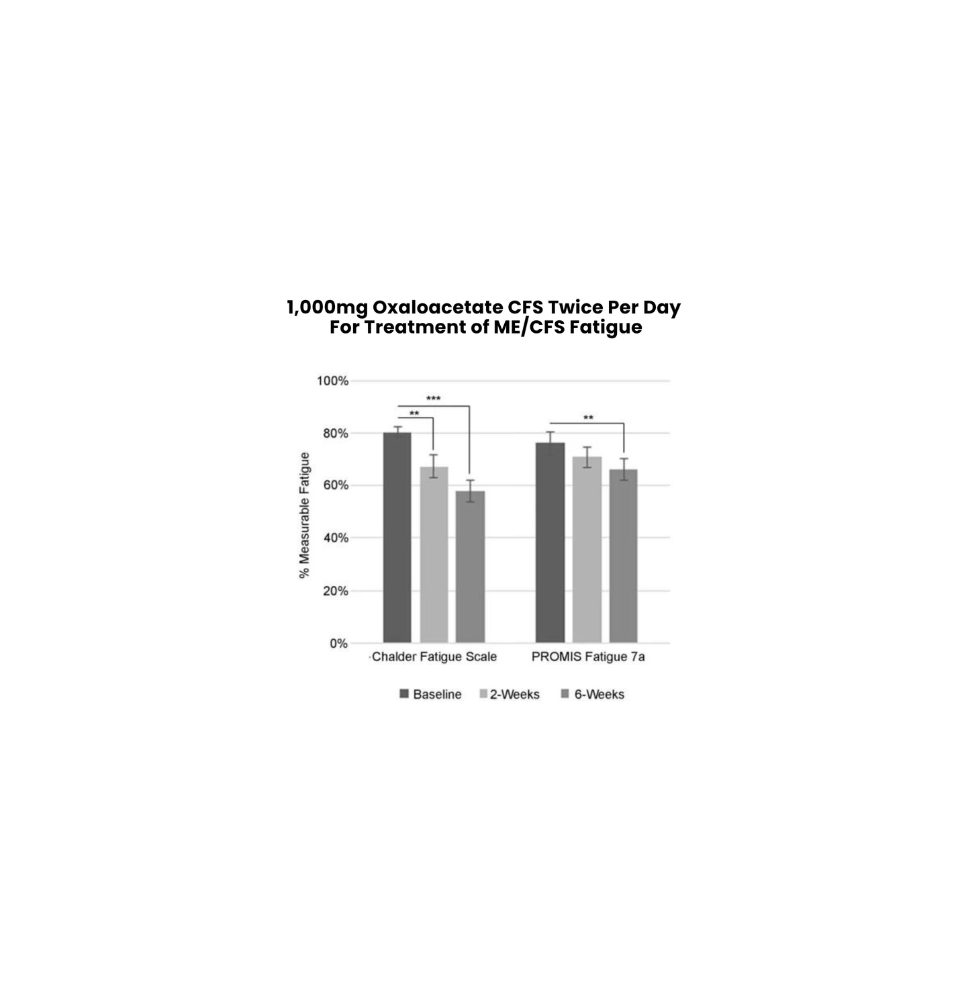
Clinically Proven To Lower Fatigue
Oxaloacetate CFS is clinically proven to lower fatigue in the latest study peer-reviewed and published by Springer Nature’s Journal of Translational Medicine.
In the first 6 weeks of daily Oxaloacetate CFS…
- Chronic Fatigue Syndrome patients showed a reduction in fatigue by up to 33.3%
- Long COVID patients showed a reduction in fatigue by up to 46.8%
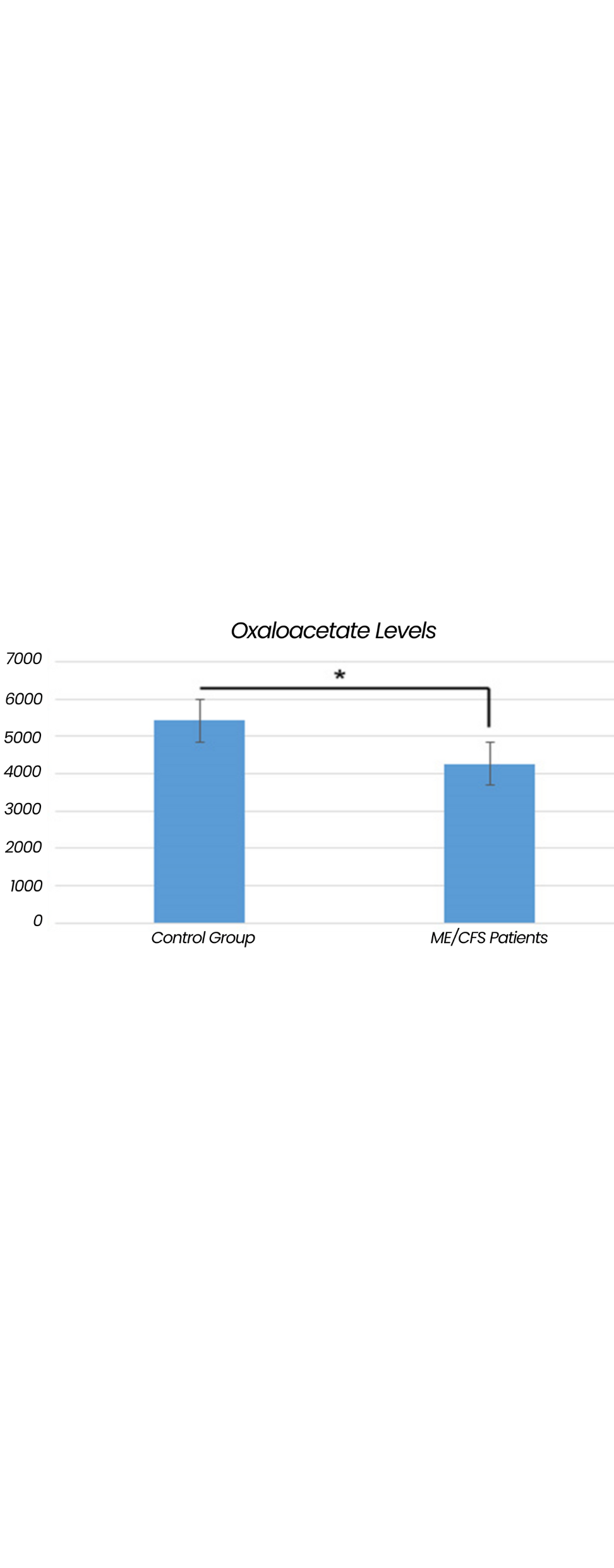
CFS Patients Have Low Oxaloacetate Levels
Metabolomic studies in ME/CFS patients vs. normal controls indicate that oxaloacetate levels are significantly reduced in the plasma of ME/CFS patients.
The Journal of Molecular Biosystems published a 2017 clinical trial showing a statistically significant shortage of oxaloacetate in ME/CFS patients compared to a normal control group
Ordinary foodstuffs do not contain enough oxaloacetate to overcome this shortage, so the dietary management of ME/CFS patients cannot be achieved by the modification of the normal diet alone.
What is A Medical Food?
-

Medical Foods
Are always to be used only under the supervision of a licensed healthcare provider. Provide support for the nutritional management of a specific disease or condition that has distinct and established nutritional requirements. Support those who have trouble digesting and absorbing food and certain nutrients and cannot nutritionally manage their condition through diet modification alone.
-

Dietary Supplements
Provide supplementary nutritional support for healthy people. Are intended to affect the normal structure or function of the human body. While benaGene is a dietary supplement, Oxaloacetate CFS is not.
Oxaloacetate CFS is a medical food, a classification distinct from dietary supplements.
-

Patented Biotechnology made in the USA
-

Tested in Peer-Reviewed Clinical Trials
-

Approved by Doctors & Health Professionals
-

Patients in More Than 20 Countries Worldwide

Oxaloacetate CFS - 90 Count Bottle
A Medical Food For Chronic Fatigue Syndrome. Oxaloacetate may help alleviate physical and mental fatigue symptoms associated with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).*
Share


New Study: Oxaloacetate Reduces Chronic Fatigue In Just Six Weeks, Promising Data For ME/CFS Patients
SAN DIEGO, June 29, 2022 — A new study reports significantly reduced mental and physical fatigue in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Long COVID patients, with the administration of oral Anhydrous Enol-Oxaloacetate, (AEO), a medical food and nutritional supplement.
The study reports that six weeks of oxaloacetate treatment resulted in,
- Reduction of fatigue in Long COVID patients by up to 46.8%
- Reduction of fatigue in ME/CFS patients by an average of 22.5% to 33%
The controlled clinical trial, using a dose escalating methodology, utilized ME/CFS patients who had been diagnosed for an average of 8.9 years and Long COVID patients with symptoms for at least 6 months.
ME/CFS patients were given oxaloacetate doses of either 500 milligrams twice per day, 1,000 milligrams twice per day or 1,000 milligrams three times per day. Reduction in fatigue was dose dependent, with the smallest dose yielding a 21.7% reduction in fatigue and the largest yielding a 33.3% reduction in six weeks. Long COVID patients were given either 500 milligrams twice per day or 1000 milligrams of oxaloacetate twice per day, with fatigue reduced by up to 46.8% in six weeks.
“This data is showing oral oxaloacetate treatment may play a key role in moving dysfunctional metabolic changes back towards normal functioning,” says David Lyons Kaufman, MD, study coauthor and founder of the Center for Complex Diseases. “We certainly owe it to ME/CFS patients to give this treatment more investigation.”
Oxaloacetate, a human energy metabolite, is present in nearly every cell of the human body. Metabolomic studies in ME/CFS patients vs. normal controls indicate that oxaloacetate levels are significantly reduced in the plasma of ME/CFS patients.
“For the millions of people worldwide struggling to live with chronic fatigue, this is really hopeful news,” says Alan Cash, coauthor of the study published by the Journal of Translational Medicine, a Springer/Nature publication. “Amelioration of fatigue to this degree may be life changing. This could look like the difference between being stuck in bed for months and being able to get back to work, attending a wedding, and enjoying life again.”
The complete peer-reviewed clinical trial results are made available by the Journal of Translational Medicine.
Oxaloacetate Treatment For Mental And Physical Fatigue In Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Long-COVID fatigue patients: a non-randomized controlled clinical trial
Collapsible content
Abstract
Background
There is no approved pharmaceutical intervention for Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome (ME/CFS). Fatigue in these patients can last for decades. Long COVID may continue to ME/CFS, and currently, it is estimated that up to 20 million Americans have significant symptoms after COVID, and the most common symptom is fatigue. Anhydrous Enol-Oxaloacetate, (AEO) a nutritional supplement, has been anecdotally reported to relieve physical and mental fatigue and is dimished in ME/CFS patients. Here, we examine the use of higher dosage AEO as a medical food to relieve pathological fatigue.
Methods
ME/CFS and Long-COVID patients were enrolled in an open label dose escalating “Proof of Concept” non-randomized controlled clinical trial with 500 mg AEO capsules. Control was provided by a historical ME/CFS fatigue trial and supporting meta-analysis study, which showed average improvement with oral placebo using the Chalder Scale of 5.9% improvement from baseline. At baseline, 73.7% of the ME/CFS patients were women, average age was 47 and length of ME/CFS from diagnosis was 8.9 years. The Long-COVID patients were a random group that responded to social media advertising (Face Book) with symptoms for at least 6 months. ME/CFS patients were given separate doses of 500 mg BID (N=23), 1,000 mg BID (N=29) and 1000 mg TID (N=24) AEO for six weeks. Long COVID patients were given 500 mg AEO BID (N=22) and 1000 mg AEO (N=21), again over a six-week period. The main outcome measure was to compare baseline scoring with results at 6 weeks with the Chalder Fatigue Score (Likert Scoring) versus historical placebo. The hypothesis being tested was formulated prior to data collection.
Results
76 ME/CFS patients (73.7% women, median age of 47) showed an average reduction in fatigue at 6 weeks as measured by the “Chalder Fatigue Questionnaire” of 22.5% to 27.9% from baseline (P<0.005) (Likert scoring). Both physical and mental fatigue were significantly improved over baseline and historical placebo. Fatigue amelioration in ME/CFS patients increased in a dose dependent manner from 21.7% for 500 mg BID to 27.6% for 1000 mg Oxaloacetate BID to 33.3% for 1000 mg TID. Long COVID patients’ fatigue was significantly reduced by up to 46.8% in 6-weeks.
Conclusions
Significant reductions in physical and metal fatigue for ME/CFS and Long-COVID patients were seen after 6 weeks of treatment. As there has been little progress in providing fatigue relief for the millions of ME/CFS and Long COVID patients, anhydrous enol oxaloacetate may bridge this important medical need. Further study of oxaloacetate supplementation for the treatment of ME/CFS and Long COVID is warranted. 1,000 mg BID Normalized Fatigue Data for Baseline, 2-weeks and 6-weeks evaluated by 3 Validated Fatigue Scoring Questionnaires
Key Points
Question: Can normalization of metabolism with oxaloacetate help reduce fatigue in ME/CFS and Long COVID?
Findings: Patients with ME/CFS and Long-COVID treated with oral Anhydrous Enol-Oxaloacetate capsules achieved highly significant reductions in physical and mental fatigue within 6 weeks.
Meaning: Pathological Fatigue is an unmet medical problem pervasive in ME/CFS, Long-COVID, and other diseases. Here, treatment to normalize metabolism with Anhydrous Enol-Oxaloacetate has for the first time shown improvements in Pathological Fatigue.
The full study data is made available by the Journal of Translational Medicine.






